hardness test of water sample|check my water hardness : wholesaling 2340 HARDNESS* 2340 A. Introduction 1. Definition Originally, water hardness was understood to be a measure of the capacity of water to precipitate soap. Soap is precipitated chiefly by . Resultado da 4 de fev. de 2019 · Caso tenha alguma dúvida, comentário ou sugestão sobre esta funcionalidade, compartilhe aqui conosco!Telefone : (21) 2276-6400
{plog:ftitle_list}
weblinkr.bio/IDT-Bogota. The All-in-One Monetization Platform for Creators and Brands! You can create your link-in-bio, grow your following, monetize creativity, and prosper with .
2340 HARDNESS* 2340 A. Introduction 1. Definition Originally, water hardness was understood to be a measure of the capacity of water to precipitate soap. Soap is precipitated chiefly by .You will then use the EDTA solution to determine the hardness of an unknown water sample. Since both EDTA and Ca2+ are both colorless, it is necessary to use a rather special indicator .As mentioned in experiment 9 of CHE-3(L) course, total hardness of water can be estimated by titrating a sample of water with EDTA salt (disodium salt of ethylene diamine tetraacetic acid) .Hardness of water is determined by titrating with a standard solution of ethylene diamine tetra acetic acid (EDTA) which is a complexing agent. Since EDTA is insoluble in water, the .
You can test for hard water by using a simple soap test, a DIY hardness test kit, or a laboratory test. If you discover hard water, you can tackle the issue by installing a water softening system in your home.In this experiment you would learn about and perform a complexometric titration to determine the hardness, of a given water sample, expressed in terms of ppm of CaCO3. In the next .
You will use EDTA complexometric titration to determine the hardness of a sample of water brought from your home. Both the total hardness and the individual calcium and magnesium .
Test methods for determining the total hardness of drinking water. Full size table. The test strips are designed for visual assessment of the total hardness in water by .Key Concepts. Calcium and magnesium salts dissolved in water cause water hardness. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid (EDTA). The ionised form of EDTA is . After you test your tap water, it’s important to know your local results to decide whether you need to filter your water, Consumer Reports says. Ad-free. Influence-free. There are several ways to test for hard water. The most accurate test is done through an independent laboratory, using a sample you provide, but a comprehensive water test can be expensive and take a while for results. .
Hardness: Water hardness refers to the presence of calcium and magnesium salts in water. Temporary hardness is caused by carbonate and bicarbonate ions, while permanent hardness is due to chloride and sulfate .Water hardness is dominated by the presence of calcium and magnesium cations because of extensive contact that water has with soil and rock . sample measurement for each hardness type and for every 20 or fewer samples analyzed. 7.0 Bibliography 1. Eugene W. Rice, Rodger B. Baird, Andrew D. Eaton, and Lenore S.Testing water hardness is important for several reasons: Water hardness can impact the performance and lifespan of appliances such as water heaters, dishwashers, and washing machines. . Dip the test strip into the water sample for a few seconds. Make sure the strip is fully submerged. Step 4: Wait for the Results .The hardness of high-quality water should not exceed 270 mg/l (15.5 grains per gallon) measured as calcium carbonate. Water softer than 30 to 50 mg/l may be corrosive to piping, depending on pH, alkalinity and dissolved oxygen. Water softeners will correct hard water of more than 270 mg/l.
Here’s how to test your water using a DIY water test kit: Take a drinking water sample from your faucet in a clean cup or container; . Hach Total Hardness Water Test Strips; Type: Lab Test: Lab Test: Lab Test: Lab Test: Test Strips: Test Strips: Test Strips: Test Strips: Rating: 5/5: 4.5/5: 4.5/5: 4/5: 4/5: 4/5: 4/5: 4/5: Analytes Tested:
The first step to solving hard water problems is to implement a water hardness test so you can find the best water softener solution. Here are a few ways to get started: . iron, nitrates and/or pH. To obtain the most thorough water analysis, you can send a sample to a third-party lab, which can test for specialty contaminants such as heavy . SAMPLE CUP INCLUDED - Ensure the most accurate water hardness test results by using the included, free sample cup and fill the testing water to the marked level. 200 WATER TEST STRIPS @ Cool to < 10 °C (< 50 °F) for source water and groundwater samples (recommended for drinking water as well) but do not allow samples to freeze . Holding Times Holding times are generally very short - 8 hours for source water compliance samples, 30 hours for drinking water samples, 48 hours for coliphage samples. Deliver samples to.04 EACH - Maximize the time between each purchase with the lowest cost-per-test water hardness test strips that are available.Rinse a 10.0 mL pipette with some of the hard water sample then pipette 10.0 mL of the hard water into a clean conical flask. Use a clean conical flask that has been rinsed with deionised water. Add 5 mL of ammonia based pH 10 buffer using a pipette 1. Learn how to test water hardness at home using kits, soap tests or electronic meters, and get actionable steps to soften hard water and protect your plumbing.
2. Water Hardness Test Kits (DIY) For a more precise test, you can use any water hardness kit. You purchase one online or from local stores at a small cost. Three different types of hard water test kits include; Test strip – The test strips usually come with a color chart showing the classification of water hardness. Fill a glass with some . How can I know the source of the sample in the water hardness? Reply. Kunika says: . Determination of hardness of water by EDTA method is one of the three main methods for determination of hardness of water. With calcium and magnesium ion, this reagent can forms a stable complex. . 2013 hard water, Quality test determination of hardness of .Total Water Hardness =[CaCO 3] = 2.5 * 24 mg/L + 4.1 * 28 mg/L = 174.80 mg/L The result is the total hardness of water: 174.80 mg/L or 174.80 ppm. If you have such a high water hardness, you might want to consider using a salt-free .First, you have to measure the water hardness with a water hardness test. You can get it online for less than . If you measure that your water hardness is below 60 ppm or 3.50 GPG, you don’t have to worry about hard water. By .
At Pentair Water Solutions, we connect customers with a water test. Simply collect your tap water sample and send it back to us via a prepaid label. Our water testing kits will measure your water hardness and test your water for .Estimation of Hardness of Water by EDTA Method 3 In the pH range 8-10, the blue form of the indicator HD2– gives a wine red complex with Mg2+: Mg+2 + HD 2– MgD– + H+ (Blue) (Wine red) Now if EDTA (H2Y 2–) is added to such a solution Mg2+ preferentially complexes with EDTA (since the metal EDTA complex is more stable than the metal-indicator complex) and liberates .
Alkalinity or Acid Neutralizing Capacity (ANC) Purpose: The purpose of this assignment is to introduce concepts of titrimetry as they pertain to the determination of ANC and water hardness of water samples. Learning Objectives: At the end of this assignment, students will be able to:. Understand the chemistry occurring in the analysis of ANC. Be able to .
An accurate water test is the first step to protecting plant, animal and human health. . Chloride, Fluoride, Iron, Magnesium, Nitrate, Potassium, Sodium, Sulfate, Total Hardness (CaCO3), Total Alkalinity (CaCO3), Electrical Conductivity, pH, Est. Total Dissolved Solids, Cation/Anion Balance . If submitting a water sample from outside the US .
A major application of EDTA titration is testing the hardness of water, for which the method described is an official one. . Dilute 25 mL tap water sample (or such volume as to require <15 mL titrant) to ca. 50 mL with dd water in 125-mL Erlenmeyer flask. For tap distilled water, test 50 mL, without dilution. Prepare samples in triplicate.
Hard Water: Hard waters are generally considered to be those waters that require considerable amounts of soap to produce foam and that also produce scale in water pipes, heaters, boilers and . Ca-Hardness: Take 50 ml of the sample and add 1 ml Sodium Hydroxide solution (8%) in it and add pinch of Mercurex Powder. Titrate with standard EDTA .For testing contaminants like bacteria, you must make sure the faucet does not contaminate the water sample. To ensure a clean sample, wipe the entire water outlet area of the faucet and spigot with a solution of 1/8 teaspoon household bleach in 12 ounces of tap water.
Recommended for targeted testing of hardness level in drinking water. Reported results will include detailed, quantified analysis of hardness by SM 2340B (or equivalent). . Your kit includes all the bottles, tubes or vials necessary to collect your water sample. For most kits, however, there is only one collection bottle needed. .
Paper core Crush Tester distribute
The biggest drawback with drop-based hard water test kits is they are more difficult to use. They also can be as much as 3 times more expensive than hard water kits that use test strips. Among the drop-based water hardness test kits that I like the most is the ‘Hach 145300 Total Hardness Test Kit, Model 5-B’.For example, if a water sample is found to have a Ca 2+ concentration of 30mg/L, then its calcium hardness as CaCO 3 can be calculated using the formula (30 mg/L Ca 2+) × (100 g CaCO 3 / 40 g Ca 2+) . See Test 14, Total Water Hardness, for further information about this topic. Objectives.
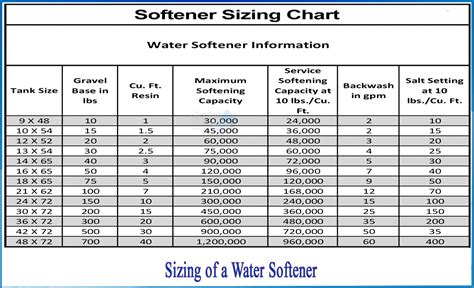
water softener hardness chart
water hardness testing near me

Belvedere Encantado. Aqui o visitante desfrutará de uma das belas paisagens da região, o mirante da ferradura do rio Taquari entre os Municípios de Encantado, Muçum e Roca .
hardness test of water sample|check my water hardness